Current State of Art After Twenty Years of the Discovery of Bioactive Peptide Lunasin

Potential Health Benefits Associated with Lunasin Concentration in Dietary Supplements and Lunasin-Enriched Soy Extract
1
Section of Food Science and Human Diet, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
2
Tecnología Alimentaria, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A. C., CIATEJ, Guadalajara 44270, Mexico
three
Center for Crops Utilization Research, Iowa State Academy, Ames, IA 50011, The states
*
Author to whom correspondence should be addressed.
†
Currently located at Department of Food Science, Academy of Tennessee, Knoxville, TN 37996, The states.
Academic Editors: Carmen Lammi and Yula Sambuy
Received: 20 April 2021 / Revised: 6 May 2021 / Accustomed: 7 May 2021 / Published: 12 May 2021
Abstract
Lunasin has demonstrated antioxidative, anti-inflammatory, and chemopreventive backdrop. The objectives were to evaluate the concentration of lunasin in unlike lunasin-based commercial dietary supplements, to produce a lunasin-enriched soy extract (LESE) using a ii-stride pilot-plant-based ultrafiltration procedure, and to evaluate their biological potential in vitro. LESE was produced using xxx and 1 kDa membranes in a custom-made ultrafiltration skid. Lunasin was quantified in eight products and LESE. Lunasin concentrations of the lunasin-based products ranged from 9.2 ± 0.six to 25.7 ± i.1 mg lunasin/g poly peptide. The LESE extract independent 58.two mg lunasin/g poly peptide, up to 6.3-fold higher lunasin enrichment than lunasin-based dietary supplements. Antioxidant capacity ranged from 121.five mmol Trolox equivalents (TE)/g in At present® Kids to 354.four mmol TE/g in LESE. Histone acetyltransferase (Lid) inhibition ranged from 5.three% on Soy Sentials® to 38.three% on synthetic lunasin. ORAC and lunasin concentrations were positively correlated, and Chapeau and lunasin concentrations were negatively correlated (p < 0.05). Melanoma B16-F10 and A375 cells treated with lunasin showed dose-dependent inhibitory potential (IC50 equivalent to 330 and 370 μM lunasin, respectively). Lunasin showed poly peptide kinase B expression (57 ± 14%) compared to the control (100%) in B16-F10. Lunasin concentration plant in commercial products and lunasin-enriched soy excerpt could exert benefits to consumers.
1. Introduction
Lunasin is a 40-3 amino acid peptide originally isolated from soybeans. Information technology features a unique amino acid sequence containing an arginine-glycine-aspartic acid cell adhesion motif and a polyaspartic acid tail on its carboxylic acrid end [i,2,3,4,v,6,7,viii,9,10,eleven,12]. The internalization of lunasin in macrophages is primarily mediated by endocytic mechanisms that involve integrin signaling, clathrin-coated structures, and micropinosomes. The aggregation of the clathrin-coated structures and the punctate localization of lunasin at the intracellular sites could bespeak lunasin endocytosis and internalization into the nucleus via nucleolar sequestration [13].
Lunasin exhibits different biological and chemopreventive backdrop including anti-inflammatory, anticarcinogenic, antioxidant and immune-modulating properties, anti-atherosclerosis, and osteoclastogenesis inhibition potential [i,ii,3,iv,five,6,7,8,9,10,11,12,thirteen].
The reported anti-inflammatory property is attributed to its capability to inhibit the translocation of the transcription factor NF-κB [14] while the antioxidant holding is attributed to its potential to scavenge peroxy radicals and block Fenton reactions past chelating ferrous ions, thereby leading to protection of Dna from oxidative harm [15]. Besides, lunasin can potentiate the chemopreventive effects of other compounds, such as aspirin and anacardic acid [16]. Lunasin has shown anticancer backdrop in vivo confronting breast [17], skin [18], and colon cancers [nineteen]. Its potential application in cancer immunotherapy has been explored, through in vitro and in vivo lymphoma models, showing that the combination of lunasin with cytokines IL-12 and/or IL-2 has higher tumoricidal activity than without [7]. Furthermore, lunasin can act every bit a vaccine adjuvant in activating dendritic cell role in models of lymphoma [20]. Besides, lunasin inhibits histone H3 and H4 acetylation when using a histone acetyltransferase analysis [21].
Considering of these reported health benefits, increasing numbers of lunasin-based dietary supplements are now available on the market. Patented products containing lunasin have been commercialized in the US market place for their claimed ability to lower cholesterol and low-density lipoprotein cholesterols [22] and their claimed ability to increase leptin and adiponectin plasma levels indicating a possible role in preventing obesity [23].
Gonzalez de Mejia et al. [24] analyzed lunasin in commercial soy protein isolates and isoflavone products. Hernandez-Ledesma et al. [25] also evaluated lunasin and Bowman-Birk inhibitors in US commercially available soymilk and soy-based infant formulas using western blot. In addition, Cavazos et al. [26] analyzed lunasin concentrations in organic soymilk, soy poly peptide shakes, and infant formulas using an enzyme-linked immunosorbent assay (ELISA). On the other hand, there are patented lunasin purification methods (WO2011060181A1), this patent includes methods for an extraction solution, multiple process steps for lunasin purification including size-based filtration, accuse-based filtration, and hydrophobicity-based filtration techniques [27]. However, no studies on lunasin concentration and its biological potential in lunasin-based commercial dietary supplements accept been reported. The hypothesis of this research is that it is possible to produce, at a pilot-plant level, a lunasin-enriched soy extract (LESE) with a higher concentration of lunasin and higher bioactivity than lunasin-based commercial products. The objectives of the present study were to evaluate the concentration of lunasin in different lunasin-based commercial dietary supplements, to produce a lunasin-enriched soy extract using a two-step pilot-plant-based ultrafiltration process, and to evaluate their biological potential.
2. Materials and Methods
2.i. Materials
Defatted soybean flour was provided by Archer-Daniels-Midland Company (Decatur, IL, Usa). Lunasin rabbit polyclonal antibody was provided by Dr. Ben O. de Lumen (University of California, Berkeley, CA, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
2.2. Lunasin-Based Commercial Dietary Supplement Samples
Lunasin-based commercial samples were purchased online. Eight samples were purchased: (ane) Soy Sentials®, (ii) Slimplicity®, (3) Now®, (4) Provantage®, (5) LunaRich X™ capsule containing a pure concentrated form of lunasin, (6) GlucAffect®, (vii) Now® kids-vanilla, and (viii) Now® kids-chocolate. Ingredients for each dietary supplement are presented in Table one.
2.three. Pilot-Plant Production of Lunasin-Enriched Soy Extract
Production of lunasin-enriched soy excerpt was performed in the pilot establish of the Center for Crops Utilization Enquiry, Iowa State University (Ames, IA, USA). Defatted soy flour was suspended in deionized h2o in a i:10 ratio and mixed for xc min at room temperature. After that, the mixture was centrifuged with a Centrisys horizontal decanter (Model no. CS10-4 3PH, Kenosha, WI, United states) at 4255 rpm bowl speed, 2 rpm differential solid gyre speed, with 11.5 L/min (LPM) feed speed. The resulting supernatant was subjected to two-stage ultrafiltration using membranes with 30 kDa and i kDa molecular weight cutting-offs obtained from Sepro Membranes, Inc. (Oceanside, CA, USA). The pilot-constitute ultrafiltration system was a custom-made sideslip manufactured by MP&C, Inc. (Edgar, WI, USA). The ultrafiltration was carried out using the 30 kDa cartridge under the following conditions: a feed rate of 13 LPM, a recirculation pump outlet pressure of 30 psi, and a permeate flow rate of 1.6 LPM by adjusting the retentate/permeate ratio control valve and the recirculation pump and the high-force per unit area pump. The permeate containing molecules with molecular weight <30 kDa were collected and farther subjected to some other ultrafiltration step using 1 kDa molecular weight cut-off membrane with the following conditions: a feed rate of x LPM, a recirculation pump outlet pressure of 100 psi, and a permeate catamenia rate of ane.ii LPM by adjusting the retentate/permeate ratio control valve and the recirculation pump and the high-pressure pump. The concentrated retentate was collected and freeze-dried as the final enriched lunasin extract.
2.4. Analysis of Lunasin during Pilot-Plant Based Production of Lunasin-Enriched Soy Extract
Samples of the decanter supernatant, the retentates and permeates from both 30 and ane kDa membrane treatments were analyzed for lunasin using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-Page) and western absorb assay as previously reported [1].
ii.5. Lunasin Purification Anion Exchange Chromatography
Lunasin extract (LE) was obtained equally reported previously [26] with slight changes. Briefly, freeze-dried lunasin enriched soy extract (forty% w/5) was solubilized in 50 mL distilled water, centrifuged twice at 12,000× thousand for 10 min, and filtered through a 0.45 µm filter. A HiTrap Q HP (GE Healthcare Bio-Sciences, Uppsala, Sweden) column was used coupled with a HiPrepp 26/10 desalting pre-cavalcade (GE Healthcare Bio-Sciences, Uppsala, Sweden). Unbound proteins eluted with Tris-HCl 20 mM, pH 7.4 at a 1 mL/min flow charge per unit. Bound proteins eluted using 0.4 M NaCl. The LE sample was desalted using ultrafiltration through a 1 kDa disc and freeze-dried for in vitro analysis.
2.half dozen. Protein Concentration Measurement by Detergent-Uniform (DC) Poly peptide Analysis
The protein concentration of lunasin-based commercial products and samples from ultrafiltration production of lunasin-enriched flour were determined by microplate DC poly peptide assay (Bio-Rad Laboratories, Hercules, CA, USA) as previously reported [1] and calculated using a bovine serum albumin (BSA) standard curve (y = 0.0003x + 0.0209, R2 = 0.99).
2.7. Enzyme-Linked Immunosorbent Assay
Lunasin concentrations of lunasin-based commercial products and samples from ultrafiltration production of lunasin-enriched soy extract were determined past ELISA as previously reported [1] and calculated using a synthetic lunasin standard curve (y = 0.0076x − 0.1902, R2 = 0.96).
2.8. Antioxidant Capacity
The antioxidant capacities of all the samples were measured by the oxygen radical absorbance capacity (ORAC) assay as previously described [28] and the results were expressed as mmol Trolox equivalents (TE)/g and calculated using the generated Trolox standard bend (y = 0.1150x − 3.17, Rtwo = 0.98).
2.ix. Histone Acetyltransferase (HAT) Inhibitory Screening Assay
HAT inhibition analysis was performed according to the manufacturer protocol (Cayman Chemicals, Ann Arbor, MI, USA). The assay was conducted every bit follows: fifteen µL of assay buffer, v µL of acetyl CoA, ten µL of diluted HAT/pCAF, and five µL of diluent or samples (final concentration of 100 µg protein/mL for samples and 10 µM for synthetic lunasin) were added to wells in triplicate. The reactions were initiated by adding 20 µL of HAT peptide except for background wells and incubated in a shaker for v min at room temperature. After incubation, the reaction was stopped by adding fifty µL of Hat stop reagent to all wells and xx µL of HAT peptide was added to the background wells. After which, 100 µL of Lid developer was added to each well followed by incubating for twenty min at room temperature. The plates were read using a Synergy ii microplate reader (Winooski, VT, USA) at 340/30 nm excitation wavelength and 440/twoscore nm emission wavelength. Inhibition of Chapeau was calculated based on the full HAT initial activity well (treated with diluent only).
ii.10. Cell Cytotoxicity
B16-F10 mouse melanoma (ATCC® CRL6475™) and A-375 human melanoma ATCC® CRL-1619™) were purchased from American Type Culture Collection (Manassas, VA, U.s.a.). Cells were inoculated in 96-well plates at a confluence of i × x5 cells per well and treated with different LE concentrations (0.158 ng/mL to 3.sixteen mg/mL) for 24 h. Cells were maintained at 37 °C, 5% CO2, and 95% air. Cell viability was measured using CellTiter 96® AQueous One Solution (Promega Corporation, Madison, WI, USA) according to manufacturer protocol.
2.eleven. Protein Kinase B (Akt) Pathway Expression
Cells were treated with lunasin half-maximum inhibitory concentration (ICfifty) for 24 h, and so cell lysates were produced. The protein concentration was quantified with Bio-Rad DC poly peptide assay. Based on preliminary results obtained using Mitogen-Activated Protein Kinases (MAPK) (ab211061; Abcam, Cambridge, MA, USA) array (data not shown), Protein kinase B (Akt) (AAH-AK T-1-8; Ray Biotech, Norcross, GA, The states) was focused on using a protein concentration of 300 µg of protein/mL. The epitopes for AKT assortment are shown in Table two.
two.12. Statistical Analysis
All analyses were performed in at least ii independent replicates with 2 replicates. Information were analyzed using the proc GLM command in SAS software version 9.4. Statistical significance was reported at p < 0.05 and Tukey posthoc test was applied. GraphPad Prism 8 was used for IC50 analysis. AKT membranes were analyzed using a t-test.
3. Results and Give-and-take
iii.i. Analysis of Lunasin Concentrations in Lunasin-Based Commercial Dietary Supplements
Table 1 presents lunasin concentrations while Figure ane shows the protein profile (a) and western absorb analysis (b) of dissimilar commercial lunasin-based dietary supplements. On a serving basis, LunaRich X™ capsule had the lowest lunasin concentration, 0.2 ± 0.0 mg lunasin/serving (41.0 ± 2.1 mg lunasin/100 k pulverisation), while Now® kids-vanilla presented the highest lunasin concentration, 7.half dozen ± 0.iii mg lunasin/serving (26.two ± ane.i mg lunasin/100 g pulverisation) (Tabular array 1). Figure 1a shows that all the lunasin-based products independent mixtures of different soy proteins, indicating a simple extraction and/or purification steps during preparation. The protein profiles of the eight products comprise the major proteins found in soybeans as previously reported [29]. Figure 1b confirms the presence of lunasin in the commercial products as they show a positive reactivity towards lunasin rabbit polyclonal antibody. Studies on the concentration of lunasin in commercial soy products have been previously reported. No studies, however, evaluated lunasin concentrations in lunasin-based dietary supplements.
Cavazos et al. [26] reported that lunasin concentrations ranged from 1.6 to 22.two mg lunasin per serving in different organic soymilks, soy poly peptide shakes, and soy infant formulas. Hernandez-Ledesma et al. [30] reported that different soymilks had lunasin concentrations of 10.7 to eighteen.9 mg/100 mL of milk, which can be translated from 25.7 to 45.four mg lunasin per 240 mL serving. Our results showed that the lunasin concentrations of lunasin-based products were 0.two ± 0.0 to 7.vi ± 0.iii mg lunasin/serving (240 mL), which typically falls within the standard lunasin concentrations that can be constitute in different soymilk and soy-based babe formulas. Table 1 shows the lunasin concentration expressed as mg lunasin/100 thou product and mg lunasin/serving, every bit well every bit serving size and ingredients that can exist institute in each commercial lunasin-based dietary supplement. Previous in vitro studies take shown that equally little equally 10 µM of lunasin led to reducing pro-inflammatory markers TNF-α, IL-6, and IL-1β in lipopolysaccharide-induced macrophages [14,25]. Considering the low bioavailability of lunasin and a total claret volume of five L, approximately 250 mg of lunasin is needed to accomplish the 10 µM lunasin concentration. Based on the lunasin concentrations in different lunasin-based commercial dietary supplements, approximately 33 servings (At present® Kids-Vanilla) to 1250 servings (LunaRich Ten™ capsule) would exist needed to accomplish this concentration. These estimates assume that lunasin is 100% bioavailable. Previous studies on the bioavailability and digestibility of lunasin showed that 97% of lunasin is digested afterwards pepsin-pancreatin digestion [31] and only 4.5% of the remaining lunasin can exist absorbed [32] indicating that a larger number of servings of these products is needed in order to reach an constructive concentration of x µM. Continuous intake of soy-based products, however, may lead to sustained lunasin concentrations in plasma, which might be responsible for wellness benefits associated with soy consumption.
iii.two. Pilot-Plant Production of Lunasin-Enriched Soy Extract
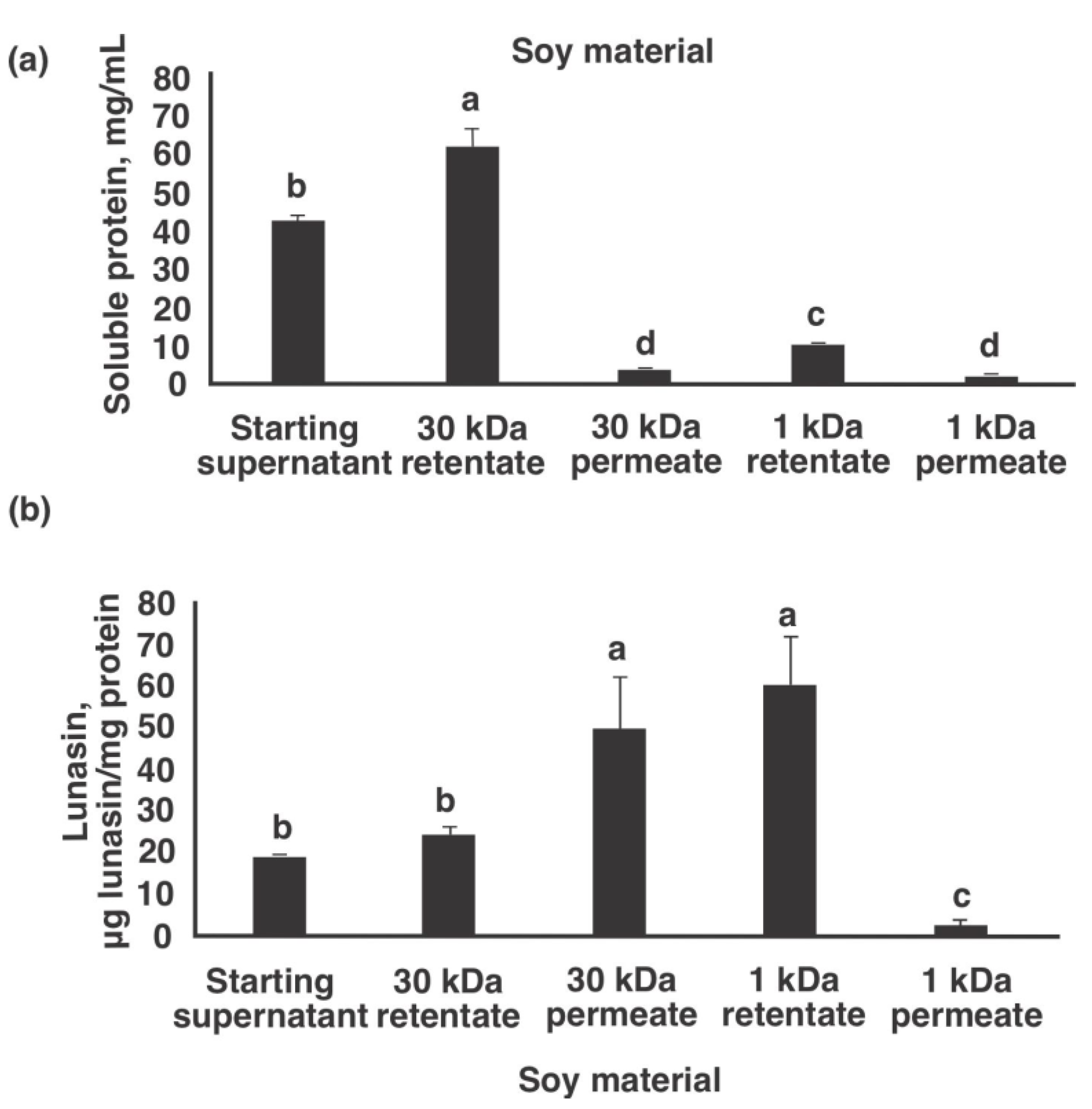
Figure 2a,b show the soluble protein and lunasin concentrations of soy materials at different stages of production of lunasin-enriched soy extract, respectively. The protein concentration of the starting supernatant subsequently centrifuging the defatted soy flour:water mixture (1:10 ratio) was 42.6 mg/mL. Later 30 kDa ultrafiltration, the retentate had an increased protein concentration to 62.5 mg/mL, which is expected equally molecules <30 kDa passed through the xxx kDa membrane cartridge. The increase in total soluble protein tin too be attributed to the concentration of the starting supernatant to 30 kDa retentate. On the other hand, the poly peptide concentration of the xxx kDa permeate was 3.5 mg/mL, at to the lowest degree 12x lower than the starting supernatant. This could be attributed to dilution of this fraction as more water passed through the thirty kDa filter. Following ultrafiltering with the 30 kDa membrane, the permeate was nerveless and further concentrated using a one kDa membrane cartridge to remove low-MW compounds including sugars and salts. The protein concentration of the resulting 1 kDa retentate increased to 10.iv mg/mL, a 3-fold increase from the starting thirty kDa permeate. Equally expected, the poly peptide concentration of the 1 kDa permeate was low at 2.1 mg/mL. The starting supernatant had a lunasin concentration of 18.2 ± 1.five µg lunasin/mg protein, which was not statistically different (p > 0.05) from the lunasin concentration of the thirty kDa retentate (24.1 ± two.eight µg lunasin/mg poly peptide). On the other hand, the lunasin concentration of the thirty kDa permeate increased to 53.0 ± xi.0 µg lunasin/mg poly peptide. The lunasin concentration of the 1 kDa retentate was similar to the xxx kDa permeate at 58.two ± 11.4 µg lunasin/mg protein. This observation was expected as the thirty kDa permeate was only full-bodied to obtain the i kDa retentate.
From the starting concentration of 18.2 µg lunasin/mg protein in the decanted supernatant, the final 1 kDa retentate with lunasin concentration of 58.2 µg lunasin/mg protein presented a 3.2-fold enrichment of lunasin concentration. To farther validate the presence of lunasin on the unlike soy fractions prepared during the two-step ultrafiltration procedure, the protein contour of each fraction was evaluated during the lunasin-enriched soy excerpt product. Figure 3a shows the electrophoretic gel profiles of the different fractions. Starting from the defatted soy flour:water mixture (lane ane), supernatant afterwards centrifugation (lane 2), 30 kDa retentate (lane 3), xxx kDa permeate (lane iv), and ane kDa retentate (lane 5), an approximately five kDa ring was stained with SimplyBlue stain, which corresponded to the ring of synthetic lunasin (lanes 6 and 7). The identities of lunasin in these fractions were further validated by the western blot profiles of the samples indicating positive reactivity towards lunasin rabbit polyclonal antibody (Figure 3b). Several reports have been done on the isolation and purification of lunasin from defatted soy flour. For instance, Seber et al. [33] used a combination of anion-exchange chromatography, reduction technique, ultrafiltration, and reverse-phase chromatography to obtain a >99% lunasin purity. While Park et al. [34] used a combination of ion-substitution chromatography and ultrafiltration techniques to obtain purified soy lunasin. Krishnan and Wang [35] reported a method to enrich lunasin based on the extraction of soybean flour with 30% ethanol followed past preferential precipitation of lunasin and protease inhibitors using calcium. This procedure yields three.2 k of lunasin and protease inhibitors from 100 g of soybean flour. The present written report is the get-go on the production of lunasin-enriched soy extract using a two-step ultrafiltration technique. The concentration of lunasin in our prepared LESE was 58.2 µg lunasin/mg protein; compared to the concentrations in commercial lunasin-enriched products (concentrations ranging from 9.ii to 25.7 µg lunasin/mg poly peptide) this process enriched lunasin by 2.3 to 6.three-fold.
3.3. Antioxidant Chapters and Inhibition Potential of Histone Acetyltransferase (Hat)
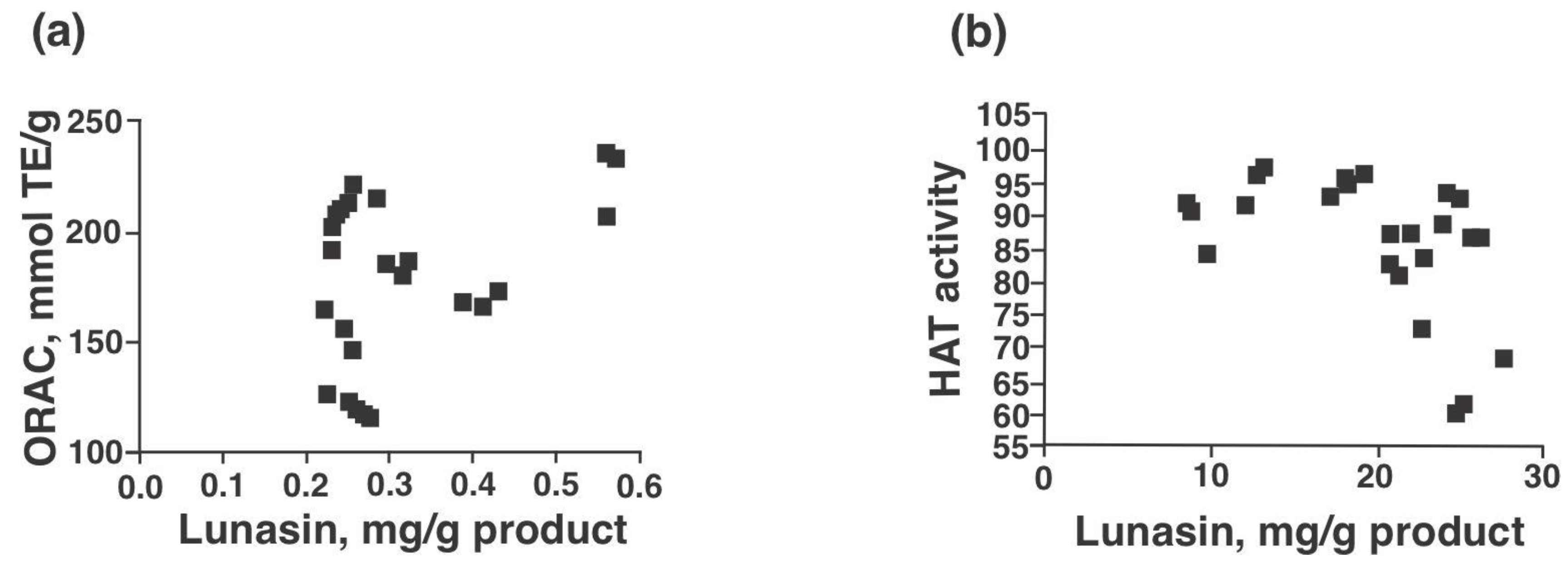
Effigy 4a,b present the antioxidant capacity (ORAC) and Lid inhibitory potential of lunasin-based dietary supplements as well as lunasin-enriched soy excerpt and constructed lunasin. The ORAC values of the samples ranged from 121.five mmol TE/1000 product for Now® kids-chocolate to 354.iv mmol TE/m for freeze-dried lunasin enriched soy excerpt (Effigy 4a). HAT inhibition ranged from v.3% for Soy Sentials® to 38.3% for synthetic lunasin (10 µM) (Figure 4b). Significant positive correlation was found betwixt ORAC and lunasin concentration of lunasin-based dietary supplements (Effigy 5a, p = 0.046, Pearson r = 0.412) and meaning negative correlation between HAT activeness and lunasin concentration of lunasin-based dietary supplements (Effigy 5b, p = 0.025, Pearson r = −0.456). Results betoken that the antioxidant capacity and the inhibition potential of Hat may be partially attributed to lunasin present in the unlike soy products. Nonetheless, phytochemicals such every bit saponins, isoflavones, amongst others could also exist present and exert an effect on the antioxidant chapters. Previous studies have shown the antioxidant potential of lunasin in different in vitro models including RAW 264.7 macrophages, HepG2 cells, and Caco-2 cells [6,fifteen,29]. On the other manus, studies on the chemopreventive property of lunasin take focused on its ability to alter the histone acetylation/deacetylation process. Lunasin in vitro is an inhibitor of H4 acetylation past p300/cAMP response element-binding poly peptide-associated factor depending on the position of lysine-acetylated [36].
iii.four. Jail cell Cytotoxicity
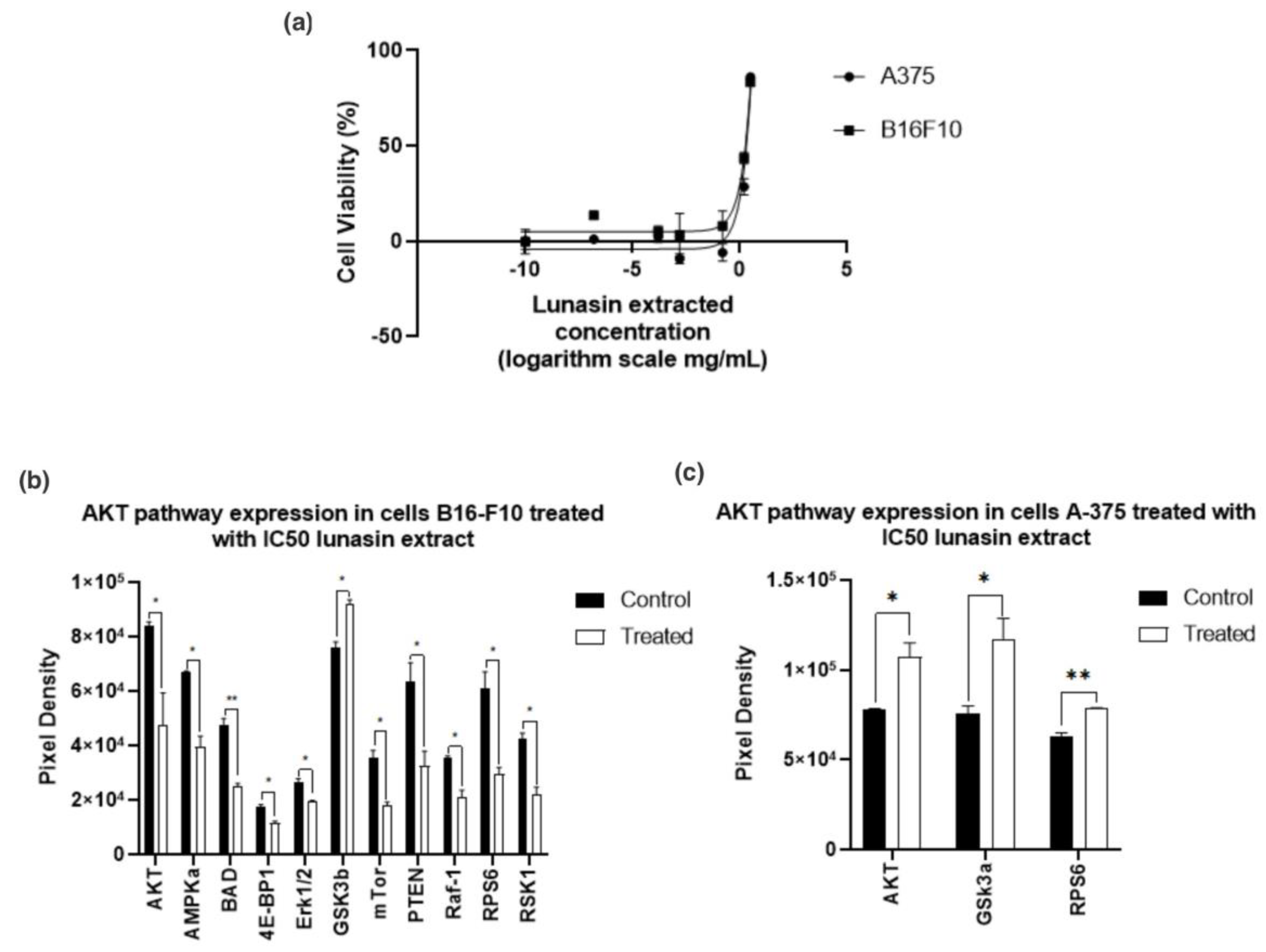
Figure 6a shows jail cell viability of B16-F10 and A-375 subsequently LE treatment. Results showed that lunasin is effective in a concentration-dependent manner. Lunasin had an IC50 = 1.84 ± 0.06 mg/mL (equivalent to 330 μM) after 24 h on B16-F10 and IC50 = 2.03 ± 0.05 mg/mL (equivalent to 370 μM) on A-375. Shidal et al. [37], reported lunasin to be effective in A-375 cells after 36 h handling with 100 μM lunasin; all the same, fifty% cell inhibition was not achieved in this study. Lunasin was showed to exist more effective in decreasing jail cell viability in A-375 melanoma cells afterwards 24 h than oleuropein a bioactive compound from olive leaves, which needed 800 μM to decrease ~fifty% after 24 h [38].
In non-melanoma prison cell lines treated with lunasin the reported IC50 < 100 μM equivalent to <550 μg/mL after 24 h [31,39,40]. Jia et al. [41] treated synovial fibroblasts with lunasin upwards to 200 μM, afterwards 48 h the IC50 was 153.iii ± 3.2 μM. After 24 h treatment, notwithstanding, lunasin was not able to inhibit 50% of jail cell viability.
3.5. Protein Kinase B (Akt) Pathway Expression
The most common mutation in melanoma is located on the BRAF gene [42,43] which activates sequentially mitogen-activated protein kinase (MEK 1/ii) and extracellular signal-regulated kinase (ERK1/2) leading to jail cell proliferation [42]. Previous studies reported that lunasin decreased phosphorylated (p-)ERK [xix,44], we hypothesized that the cell growth inhibition could be due to an ERK inhibition. After 24 h handling with IC50 in B16-F10 and A-375, the expression of p-AKT pathway (Figure 6) showed pregnant ERK inhibition (p < 0.05) for B16-F10. The inhibition of PRAS40 produced inhibition in mTOR, ribosomal protein S6 (RPS6), ribosomal poly peptide S6 kinase (P70S6k), and eukaryotic translation initiation factor 4E binding poly peptide 1 (4EBP1) interfering with jail cell growth and metabolism [45]. Lv et al. [45] reported that melanoma with high AKT activity as well showed an increase in phosphorylated PRAS40.
Since p-glycogen synthase kinase iii (GSK3) was significant in B16-F10 (p < 0.005) and A375 (p < 0.05) (Effigy half-dozen), our data suggested that lunasin promotes GSK3β inhibition through Ser9 phosphorylation. GSK3 functions in unlike cell processes including glycogen metabolism, proliferation, differentiation, movement, and survival. It could human activity equally a tumor promoter or tumor suppressor [46,47,48]. GSK3β requires phosphorylation at Tyr216 for full action, whereas, phosphorylation in Ser9 will result in an inactivation, which is the most important regulatory mechanism [46,48]. There is evidence that GSK3β inhibition limits motility and invasion of melanoma cells, which could pb to limit the metastatic beliefs via Northward-cadherin signaling inhibition [49]. In an in vivo study where mice were fed with a soy powder mixture containing 52.six% of protein, 30.ane% carbohydrates, and 5% lipids, inhibition on N-cadherin was shown [50]. Further studies to investigate whatever relationship between lunasin and N-cadherin signaling are needed.
As shown in Figure 6 and Table 2, AKT and RPS6 expressions were college than the control for A-375 in 37% and 24%, respectively. A non-significant 24% overexpression in BAD compared with the command, however, was shown in the same cell line, which suggests that AKT and RPS6 overexpression, in this instance, could non affect the prison cell growth.
4. Conclusions
The concentration of lunasin in different commercial lunasin-based dietary supplements was comparable to commercial soymilk and soy-based infant formulas. A simple two-step pilot-constitute ultrafiltration process to generate lunasin-enriched soy extract was developed. This process is an alternative for enriching lunasin content in soybean products. Moreover, a significant positive correlation betwixt ORAC and lunasin concentrations and a meaning negative correlation betwixt HAT activities and lunasin concentrations were found. Lunasin concentration in lunasin-based commercial dietary supplements and lunasin-enriched soy extract could increase the potential health benefits associated with soy consumption. Lunasin cytotoxicity in both melanoma cells was dose-dependent reaching a one-half-maximum inhibitory concentration at a concentration equivalent to 330 μM for B16-F10 and 370 μM for A375.
Author Contributions
Conceptualization, E.G.d.M.; Data curation, E.D.C.-R. and Five.D.; Formal assay, E.D.C.-R., Fifty.Chiliad., H.W. and V.D.; Funding conquering, E.G.d.M., Fifty.A.J. and T.Due west.; Investigation, East.Thousand.d.M.; L.M.; East.D.C.-R. and Five.D.; Methodology, E.D.C.-R., H.W. and V.D.; Project administration, East.Thou.d.M., L.A.J. and T.West.; Resources, Due east.G.d.Thou., T.W. and L.A.J.; Visualization, Due east.G.d.M., E.D.C.-R., L.1000., and V.D.; Writing—original draft, 5.D., East.D.C.-R. and L.M.; Writing—review & editing, E.Yard.d.Chiliad., E.D.C.-R., L.A.J., H.W., T.W. and V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by USDA-HATCH (grant number 1017440).
Institutional Review Board Statement
Non applicative.
Informed Consent Statement
Non applicable.
Data Availability Argument
Information is independent inside this article.
Acknowledgments
To Subhiksha Chandrasekaran for her assist with jail cell treatments.
Conflicts of Interest
The authors declare no conflict of involvement.
References
- Dia, Five.P.; Wang, W.; Oh, V.50.; de Lumen, B.O.; Gonzalez de Mejia, E. Isolation, Purification and Characterisation of Lunasin from Defatted Soybean Flour and in Vitro Evaluation of Its Anti-Inflammatory Activity. Nutrient Chem. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Pabona, J.Thou.P.; Dave, B.; Su, Y.; Montales, M.T.Eastward.; de Lumen, B.O.; Gonzalez de Mejia, Due east.; Rahal, O.M.; Simmen, R.C.M. The Soybean Peptide Lunasin Promotes Apoptosis of Mammary Epithelial Cells via Consecration of Tumor Suppressor PTEN: Similarities and Distinct Actions from Soy Isoflavone Genistein. Genes Nutr. 2013, viii, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Indiano-Romacho, P.; Fernández-Tomé, S.; Amigo, 50.; Hernández-Ledesma, B. Multifunctionality of Lunasin and Peptides Released during Its Simulated Gastrointestinal Digestion. Food Res. Int. 2019, 125, 108513. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.Due west. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Chemopreventive Properties of Peptide Lunasin: A Review. Poly peptide Pept. Lett. 2013, 20, 424–432. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Ramos, S.; Cordero-Herrera, I.; Recio, I.; Goya, L.; Hernández-Ledesma, B. In Vitro Chemo-Protective Outcome of Bioactive Peptide Lunasin confronting Oxidative Stress in Human HepG2 Cells. Food Res. Int. 2014, 62, 793–800. [Google Scholar] [CrossRef]
- Chang, H.C.; Lewis, D.; Tung, C.Y.; Han, L.; Henriquez, S.Thou.P.; Voiles, L.; Lupov, I.P.; Pelloso, D.; Sinn, A.50.; Pollok, Chiliad.E.; et al. Soypeptide Lunasin in Cytokine Immunotherapy for Lymphoma. Cancer Immunol. Immunother. 2014, 63, 283–295. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Wang, X. Lunasin Abrogates Monocytes to Endothelial Cells. Mol. Immunol. 2017, 92, 146–150. [Google Scholar] [CrossRef]
- Fernández-Tomé, South.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial Transport of Lunasin and Derived Peptides: Inhibitory Effects on the Gastrointestinal Cancer Cells Viability. J. Food Compos. Anal. 2018, 68, 101–110. [Google Scholar] [CrossRef]
- Bachala, D.; El-Refai, North.; Greenfield, East.; Aminoshariae, A.; Mickel, A. The Upshot of Lunasin on Receptor Activator of Nuclear Factor Kappa-B Ligand−mediated Osteoclast Formation from RAW 264.7 Cells. J. Endod. 2018, 44, 997–999. [Google Scholar] [CrossRef]
- Vuyyuri, S.B.; Shidal, C.; Davis, Grand.R. Development of the Constitute-Derived Peptide Lunasin as an Anticancer Agent. Curr. Opin. Pharmacol. 2018, 41, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Current State of Art after Twenty Years of the Discovery of Bioactive Peptide Lunasin. Food Res. Int. 2019, 116, 71–78. [Google Scholar] [CrossRef]
- Cam, A.; Sivaguru, One thousand.; Gonzalez de Mejia, East. Endocytic Mechanism of Internalization of Dietary Peptide Lunasin into Macrophages in Inflammatory Status Associated with Cardiovascular Affliction. PLoS ONE 2013, eight, e72115. [Google Scholar] [CrossRef]
- Gonzalez de Mejia, E.; Dia, V.P. Lunasin and Lunasin-like Peptides Inhibit Inflammation through Suppression of NF-ΚB Pathway in the Macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef]
- García-Nebot, Chiliad.J.; Recio, I.; Hernández-Ledesma, B. Antioxidant Activeness and Protective Effects of Peptide Lunasin confronting Oxidative Stress in Intestinal Caco-two Cells. Nutrient Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin-Aspirin Combination Against NIH/3T3 Cells Transformation Induced by Chemical Carcinogens. Plant Foods Hum. Nutr. 2011, 66, 107–113. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Hernández-Ledesma, B.; Jeong, H.J.; Park, J.H.; de Lumen, B.O. Complementary Roles in Cancer Prevention: Protease Inhibitor Makes the Cancer Preventive Peptide Lunasin Bioavailable. PLoS I 2010, five, e8890. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.; Chen, N.; Macasieb, J.; de Lumen, B.O. Chemopreventive Belongings of a Soybean Peptide (Lunasin) That Binds to Deacetylated Histones and Inhibits Acetylation. Cancer Res. 2001, 61, 7473–7478. [Google Scholar]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin Potentiates the Upshot of Oxaliplatin Preventing Outgrowth of Colon Cancer Metastasis, Binds to A5β1 Integrin and Suppresses FAK/ERK/NF-ΚB Signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef]
- Tung, C.Y.; Kyazike, S.; Lewis, D.; Han, L.; Kolb, A.; Pelloso, D.; Sinn, A.; Pollok, K.E.; Srivastava, S.; Robertson, M.J.; et al. Activation Of Natural Killer Cells By Soypeptide Lunasin and Cytokine: Implication In Cancer Immunotherapy For Lymphoma. Claret 2013, 122, 1042. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jeong, J.B.; Kim, D.Due south.; de Lumen, B.O. Inhibition of Core Histone Acetylation by the Cancer Preventive Peptide Lunasin. J. Agric. Nutrient Chem. 2007, 55, 632–637. [Google Scholar] [CrossRef]
- Galvez, A.F. Products and Methods Using Soy Peptides to Lower Full and LDL Cholesterol Levels. U.Due south. Patent 8598111 B2, 3 December 2013. [Google Scholar]
- Galvez, A.F.; Schmidt, R.; Hastings, C. Products and Methods Using Lunasin-Enriched Soy Excerpt Mixtures to Reduce Free Fat Acid Levels, Increase Leptin Levels and Increment Adiponectin Levels in Plasma. Patent WO2014145086 A2, xviii September 2014. [Google Scholar]
- Gonzalez de Mejia, E.; Vásconez, Chiliad.; de Lumen, B.O.; Nelson, R. Lunasin Concentration in Different Soybean Genotypes, Commercial Soy Poly peptide, and Isoflavone Products. J. Agric. Nutrient Chem. 2004, 52, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Antioxidant and Anti-Inflammatory Properties of Cancer Preventive Peptide Lunasin in RAW 264.vii Macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cavazos, A.; Morales, Eastward.; Dia, V.P.; Gonzalez de Mejia, Eastward. Analysis of Lunasin in Commercial and Pilot Establish Produced Soybean Products and an Improved Method of Lunasin Purification. J. Food Sci. 2012, 77, C539–C545. [Google Scholar] [CrossRef]
- Davis, G.; Barnett, B.; Cai, J.; Mcconnell, E. Lunasin-Containing Circuitous and Purification of Lunasin from Plants. Patent WO2011060181A1, nineteen May 2011. [Google Scholar]
- Prior, R.50.; Hoang, H.; Gu, 50.; Wu, X.; Bacchiocca, Chiliad.; Howard, L.; Hampsch-Woodill, 1000.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, R.; Bringe, N.A.; Gonzalez de Mejia, Eastward. Antioxidant Capacity of Alcalase Hydrolysates and Poly peptide Profiles of Two Conventional and Vii Low Glycinin Soybean Cultivars. Constitute Foods Hum. Nutr. 2010, 65, 233–240. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Lunasin and Bowman-Birk Protease Inhibitor (BBI) in US Commercial Soy Foods. Food Chem. 2009, 115, 574–580. [Google Scholar] [CrossRef]
- Gonzalez de Mejia, Eastward.; Wang, W.; Dia, V.P. Lunasin, with an Arginine-Glycine-Aspartic Acid Motif, Causes Apoptosis to L1210 Leukemia Cells by Activation of Caspase-3. Mol. Nutr. Food Res. 2010, 54, 406–414. [Google Scholar] [CrossRef]
- Dia, V.P.; Torres, S.; De Lumen, B.O.; Erdman, J.W.; Gonzalez de Mejia, E. Presence of Lunasin in Plasma of Men subsequently Soy Protein Consumption. J. Agric. Food Chem. 2009, 57, 1260–1266. [Google Scholar] [CrossRef]
- Seber, L.E.; Barnett, B.West.; McConnell, E.J.; Hume, Southward.D.; Cai, J.; Boles, Thousand.; Davis, K.R. Scalable Purification and Label of the Anticancer Lunasin Peptide from Soybean. PLoS ONE 2012, 7, e35409. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, H.J.; De Lumen, B.O. In Vitro Digestibility of the Cancer-Preventive Soy Peptides Lunasin and BBI. J. Agric. Food Chem. 2007, 55, 10703–10706. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Wang, T.T.Y. An Constructive and Simple Procedure to Isolate Abundant Quantities of Biologically Agile Chemopreventive Lunasin Protease Inhibitor Concentrate (LPIC) from Soybean. Food Chem. 2015, 177, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Relationship between Lunasin's Sequence and Its Inhibitory Activity of Histones H3 and H4 Acetylation. Mol. Nutr. Nutrient Res. 2011, 55, 989–998. [Google Scholar] [CrossRef]
- Shidal, C.; Al-Rayyan, N.; Yaddanapudi, K.; Davis, K.R. Lunasin Is a Novel Therapeutic Agent for Targeting Melanoma Cancer Stalk Cells. Oncotarget 2016, 7, 84128–84141. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, Southward.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, Fifty. Oleuropein, the Principal Polyphenol of Olea Europaea Leaf Excerpt, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Dia, 5.P.; Gonzalez de Mejia, E. Lunasin Induces Apoptosis and Modifies the Expression of Genes Associated with Extracellular Matrix and Jail cell Adhesion in Human Metastatic Colon Cancer Cells. Mol. Nutr. Nutrient Res. 2011, 55, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, West.; Bringe, Northward.A.; Berhow, Grand.A.; Gonzalez de Mejia, East. β-Conglycinins amongst Sources of Bioactives in Hydrolysates of Different Soybean Varieties That Inhibit Leukemia Cells in Vitro. J. Agric. Nutrient Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, Due south.; Yuan, H.; Chen, N. Lunasin Inhibits Cell Proliferation via Apoptosis and Reduces the Production of Proinflammatory Cytokines in Cultured Rheumatoid Arthritis Synovial Fibroblasts. Biomed Res. Int. 2015, 2015, 346839. [Google Scholar] [CrossRef]
- Zaidi, Thousand.R.; Fisher, D.E.; Rizos, H. Biology of Melanocytes and Primary Melanoma. In Cutaneous Melanoma; Balch, C.K., Atkins, M.B., Garbe, C., Gershenwald, J.Eastward., Halpern, A.C., Kirkwood, J.Yard., McArthur, One thousand.A., Thompson, J.F., Sober, A.J., Eds.; Springer International Publishing: Cham, Deutschland, 2020; pp. 3–40. ISBN 978-3-030-05070-two. [Google Scholar]
- Castañeda-Reyes, Eastward.D.; Perea-Flores, M.D.J.; Davila-Ortiz, Thou.; Lee, Y.; De Mejia, E.G. Development, Characterization and Use of Liposomes every bit Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review. Int. J. Nanomed. 2020, 15, 7627–7650. [Google Scholar] [CrossRef]
- Jiang, Q.; Pan, Y.; Cheng, Y.; Li, H.; Liu, D.; Li, H. Lunasin Suppresses the Migration and Invasion of Breast Cancer Cells past Inhibiting Matrix Metalloproteinase-2/-ix via the FAK/Akt/ERK and NF-ΚB Signaling Pathways. Oncol. Rep. 2016, 36, 253–262. [Google Scholar] [CrossRef]
- Lv, D.; Guo, L.; Zhang, T.; Huang, L. PRAS40 Signaling in Tumor. Oncotarget 2017, 8, 69076–69085. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Glycogen Synthase Kinase 3β (GSK3β) in Tumorigenesis and Cancer Chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Yard.; Liu, B.; Meng, Z.; Liu, Y.; Li, X.; Wu, Ten.; Zhou, Q.; Xu, K. MiR-26a Enhances Invasive Capacity past Suppressing GSK3β in Human Lung Cancer Cells. Exp. Jail cell Res. 2017, 352, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chao, X.J.; Wu, J.F.; Cheng, B.C.Y.; Su, T.; Fu, X.Q.; Li, T.; Guo, H.; Tse, A.K.Westward.; Kwan, H.Y.; et al. ERK/GSK3β Signaling Is Involved in Atractylenolide I-Induced Apoptosis and Jail cell Bicycle Arrest in Melanoma Cells. Oncol. Rep. 2015, 34, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- John, J.One thousand.; Paraiso, G.H.T.; Rebecca, V.Westward.; Cantini, 50.P.; Abel, E.V.; Pagano, Due north.; Meggers, Due east.; Mathew, R.; Krepler, C.; Izumi, 5.; et al. GSK3β Inhibition Blocks Melanoma Cell/Host Interactions by Downregulating N-Cadherin Expression and Decreasing FAK Phosphorylation. J. Investig. Dermatol. 2012, 132, 2818–2827. [Google Scholar] [CrossRef]
- Jheng, H.-F.; Hirotsuka, M.; Goto, T.; Shibata, M.; Matsumura, Y.; Kawada, T. Dietary Low-Fat Soy Milk Powder Retards Diabetic Nephropathy Progression via Inhibition of Renal Fibrosis and Renal Inflammation. Mol. Nutr. Food Res. 2017, 61, 1600461. [Google Scholar] [CrossRef]
Figure 1. Analysis of commercial lunasin-based dietary supplements. (a) SDS-PAGE protein electrophoretic contour and (b) Western blot analysis of different lunasin-based products. Lanes SL and MW are synthetic lunasin and molecular weight standard, respectively. Samples were used at a concentration of 100 µg protein/mL and coded as i: Soy Sentials®, 2: Slimplicity®, 3: At present® dietary supplement, 4: Provantage®, 5: LunaRich X™, six: GlucAffect®, 7: Now® kids-vanilla and 8: At present® kids-chocolate.
Figure i. Analysis of commercial lunasin-based dietary supplements. (a) SDS-PAGE poly peptide electrophoretic profile and (b) Western blot analysis of different lunasin-based products. Lanes SL and MW are synthetic lunasin and molecular weight standard, respectively. Samples were used at a concentration of 100 µg protein/mL and coded as 1: Soy Sentials®, 2: Slimplicity®, 3: Now® dietary supplement, iv: Provantage®, v: LunaRich X™, 6: GlucAffect®, 7: Now® kids-vanilla and viii: Now® kids-chocolate.
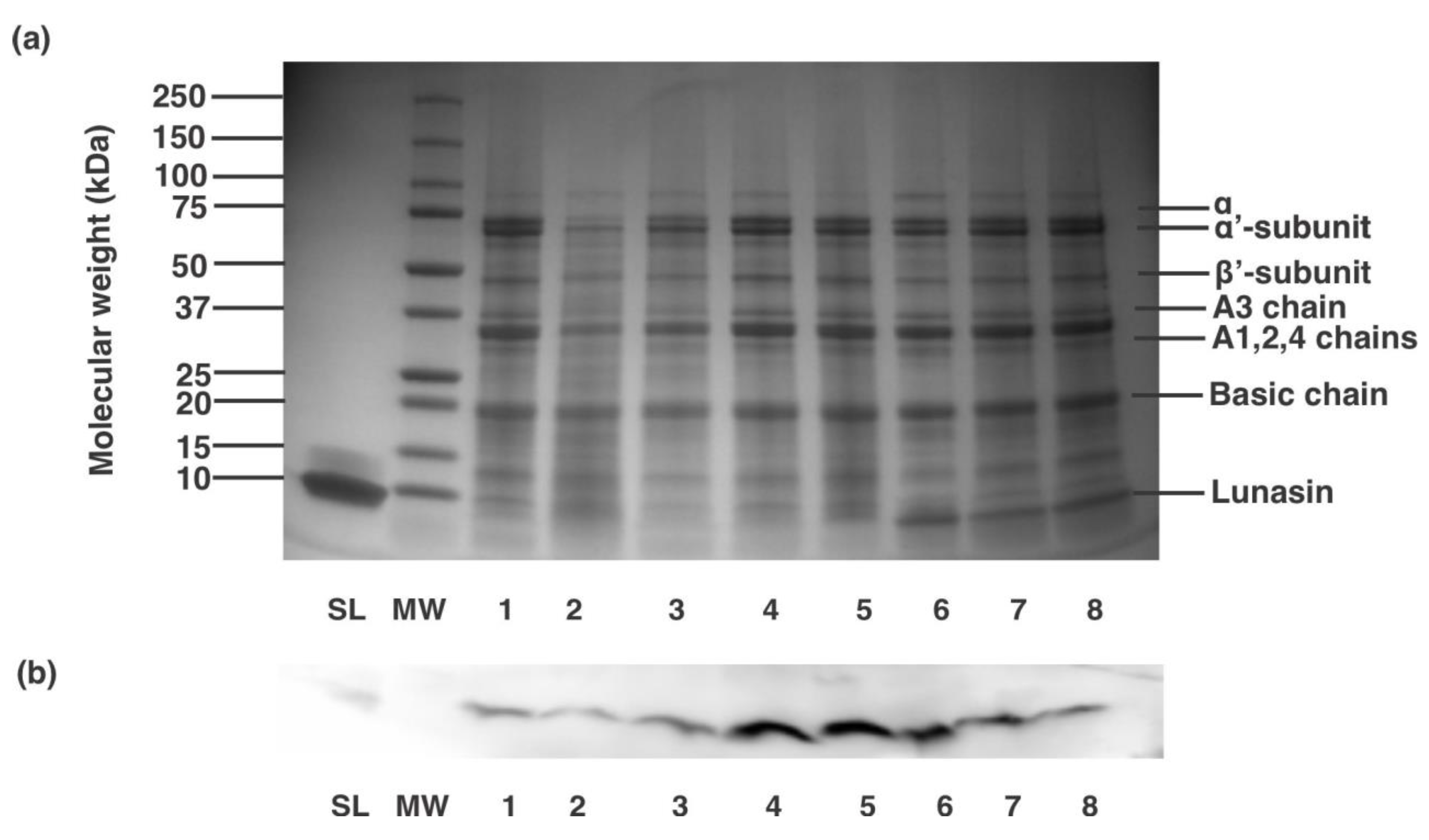
Figure 2. Soluble protein and lunasin analysis of different fractions obtained from ii-footstep ultrafiltration airplane pilot institute product of lunasin-enriched soy extract. (a) Soluble protein concentration as measured by protein DC assay, (b) Lunasin concentration as measured by ELISA. Dissimilar letters signal significant differences among fractions (p < 0.05).
Figure ii. Soluble poly peptide and lunasin assay of different fractions obtained from two-footstep ultrafiltration airplane pilot plant production of lunasin-enriched soy extract. (a) Soluble protein concentration as measured past protein DC analysis, (b) Lunasin concentration every bit measured by ELISA. Different letters indicate significant differences among fractions (p < 0.05).
Effigy 3. Poly peptide contour and lunasin identification in airplane pilot found enrich fractions. (a) Protein electrophoretic profile and (b) Western blot analysis of different fractions from pilot constitute production of lunasin-enriched soy extract. Lanes MW: molecular weight standard, 1: defatted soy flour:water mixture (one:10), 2: supernatant subsequently centrifugation, iii: xxx kDa retentate, iv: thirty kDa permeate, 5: ane kDa retentate, and half-dozen,7: constructed lunasin (10 µM).
Effigy 3. Protein profile and lunasin identification in airplane pilot constitute enrich fractions. (a) Poly peptide electrophoretic profile and (b) Western blot analysis of unlike fractions from pilot plant production of lunasin-enriched soy excerpt. Lanes MW: molecular weight standard, 1: defatted soy flour:h2o mixture (1:ten), 2: supernatant after centrifugation, 3: 30 kDa retentate, 4: 30 kDa permeate, 5: 1 kDa retentate, and vi,7: synthetic lunasin (10 µM).
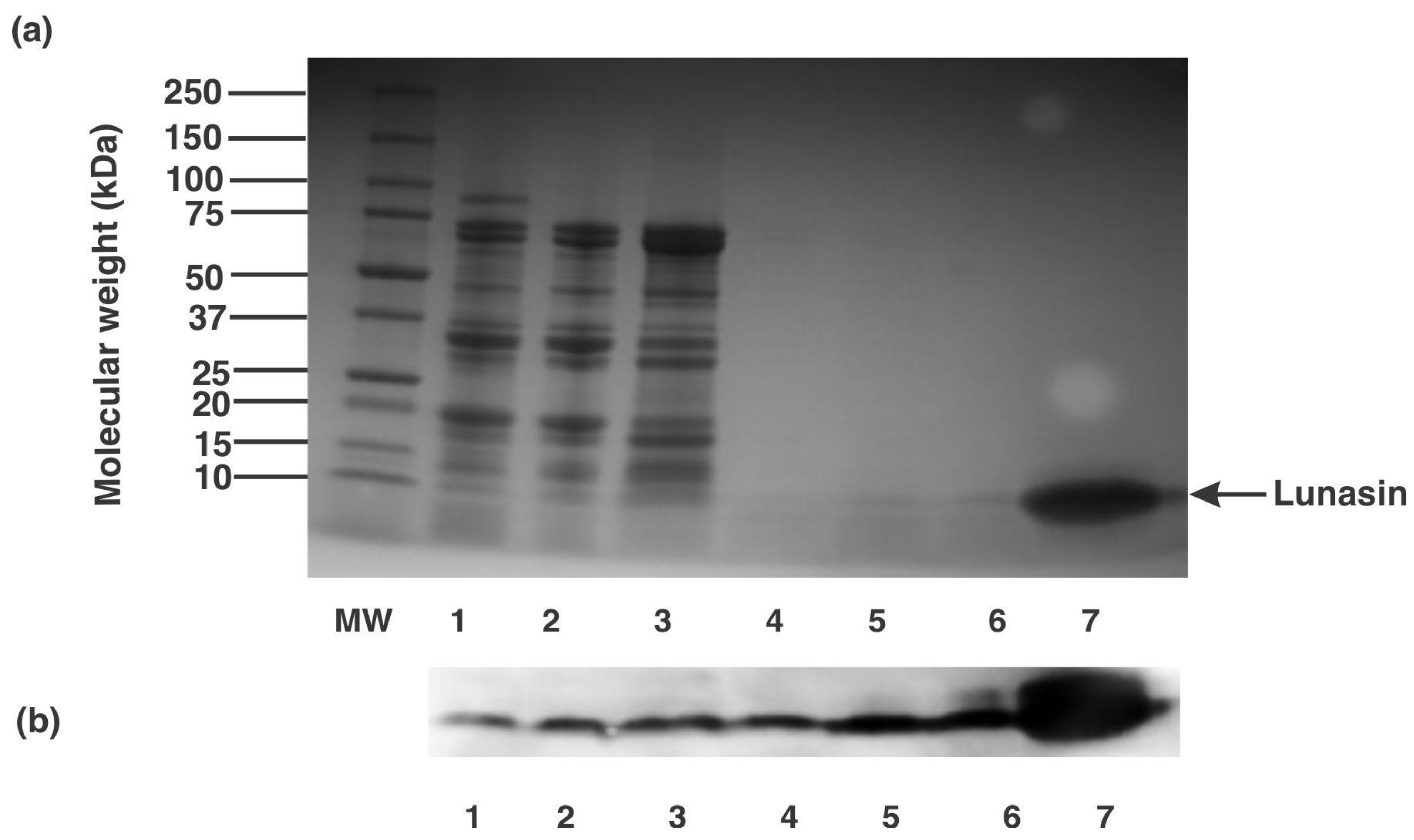
Figure 4. Biological potential of lunasin-based dietary supplements, lunasin-enriched soy extract, and synthetic lunasin. (a) Antioxidant capacity (mmol TE/g of product), (b) Histone acetyltransferase Lid (%) inhibitory potential. Samples were used at a concentration of 100 µg protein/mL and coded as 1: Soy Sentials®, ii: Slimplicity®, 3: Now® dietary supplement, iv: Provantage®, five: LunaRich X™, half-dozen: GlucAffect®, 7: Now® kids-vanilla and viii: Now® kids-chocolate, nine: Freeze-dried lunasin enriched soy extract, and ten: Constructed lunasin at 10 µM (northward = 3). Different letters point significant differences among products (p < 0.05). Gallic acid (0.1 mg/mL) had an ORAC value of 220.3 ± 2.vii mmol TE/g, used for comparison purposes.
Figure four. Biological potential of lunasin-based dietary supplements, lunasin-enriched soy extract, and synthetic lunasin. (a) Antioxidant capacity (mmol TE/g of production), (b) Histone acetyltransferase HAT (%) inhibitory potential. Samples were used at a concentration of 100 µg protein/mL and coded as 1: Soy Sentials®, two: Slimplicity®, 3: Now® dietary supplement, 4: Provantage®, v: LunaRich X™, six: GlucAffect®, 7: Now® kids-vanilla and 8: Now® kids-chocolate, 9: Freeze-stale lunasin enriched soy extract, and 10: Synthetic lunasin at 10 µM (n = 3). Different messages indicate significant differences among products (p < 0.05). Gallic acid (0.one mg/mL) had an ORAC value of 220.3 ± 2.7 mmol TE/thousand, used for comparison purposes.
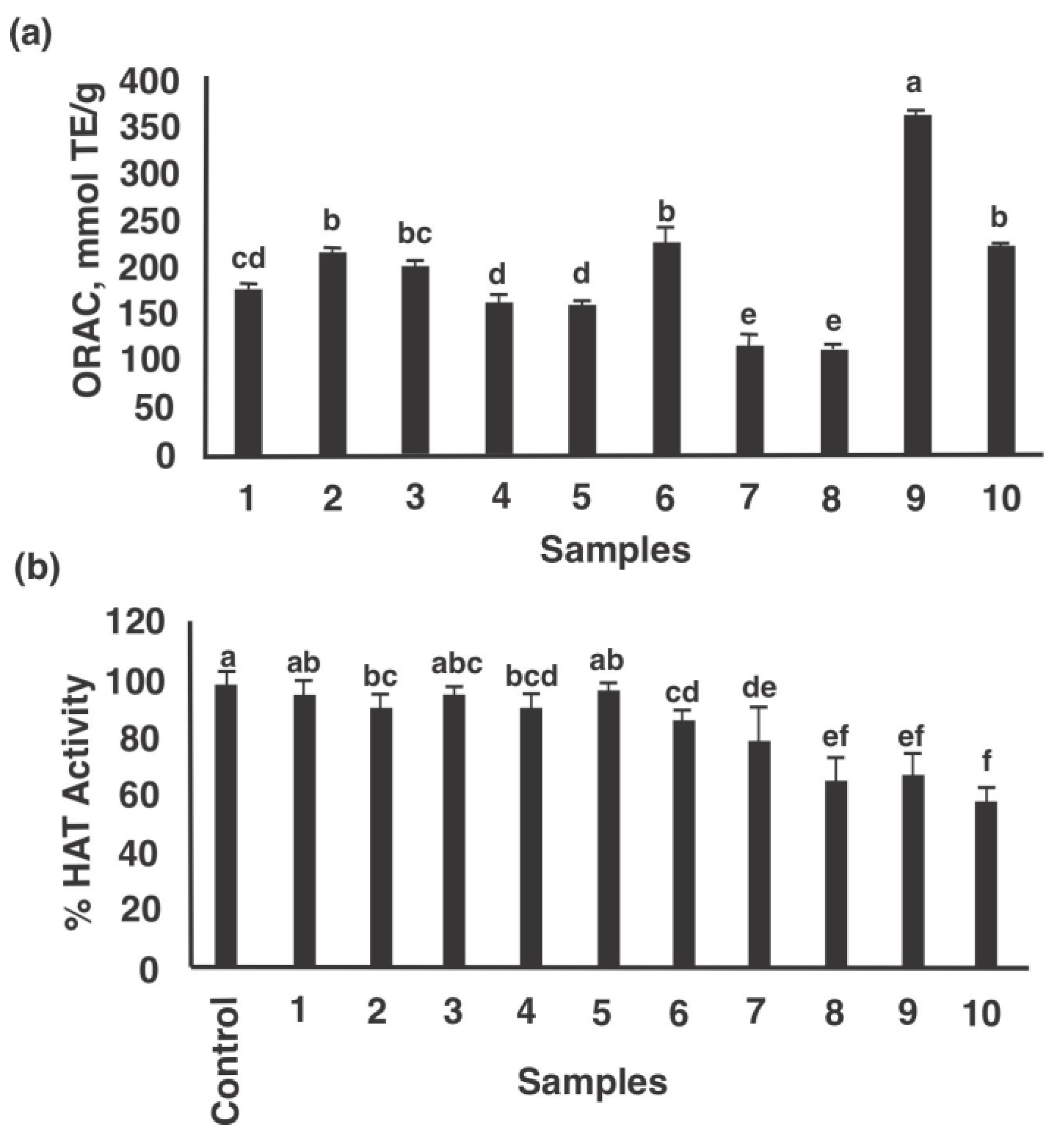
Figure five. Lunasin content correlations with antioxidant capacity and Hat inhibitory potential. (a) Lunasin (mg/g product) positively correlated with ORAC values and (b) Lunasin (mg/one thousand protein) negatively correlated with Lid activity. Samples were used at a concentration of 100 µg poly peptide/mL; materials included: Soy Sentials®, Slimplicity®, Now® dietary supplement, Provantage®, Lunarich 10™, GlucAffect®, Now® kids-vanilla and Now® kids-chocolate (northward = three). Gallic acid (0.1 mg/mL) had an ORAC value of 220.3 ± 2.seven mmol TE/k, used for comparison purposes.
Figure 5. Lunasin content correlations with antioxidant capacity and HAT inhibitory potential. (a) Lunasin (mg/g product) positively correlated with ORAC values and (b) Lunasin (mg/grand protein) negatively correlated with HAT activity. Samples were used at a concentration of 100 µg poly peptide/mL; materials included: Soy Sentials®, Slimplicity®, At present® dietary supplement, Provantage®, Lunarich X™, GlucAffect®, Now® kids-vanilla and Now® kids-chocolate (n = three). Gallic acid (0.ane mg/mL) had an ORAC value of 220.three ± ii.7 mmol TE/yard, used for comparing purposes.
Effigy vi. Melanoma cells treated with lunasin extract: (a) Jail cell viability: B16-F10 cell lines treated with unlike concentrations (0.158 ng/mL to 3.sixteen mg/mL). (b) Phosphorylated AKT pathway expression on cells B16-F10 subsequently treatment with lunasin extract ICfifty equivalent to 330 μM. (c) Phosphorylated AKT pathway expression on cells A-375 subsequently treatment with lunasin extract IC50 equivalent to 370 μM. p values reflect the comparison of the command and the treated for each marking * p < 0.05, ** p < 0.005.
Figure half dozen. Melanoma cells treated with lunasin excerpt: (a) Cell viability: B16-F10 cell lines treated with unlike concentrations (0.158 ng/mL to three.sixteen mg/mL). (b) Phosphorylated AKT pathway expression on cells B16-F10 after treatment with lunasin extract IC50 equivalent to 330 μM. (c) Phosphorylated AKT pathway expression on cells A-375 later treatment with lunasin extract IC50 equivalent to 370 μM. p values reflect the comparison of the control and the treated for each marker * p < 0.05, ** p < 0.005.
Tabular array one. Lunasin and protein concentrations of different lunasin-based dietary supplements.
Table 1. Lunasin and poly peptide concentrations of different lunasin-based dietary supplements.
| Product | Lunasin, mg/100 thousand Pulverization | Lunasin, mg/Serving | Protein *, g/Serving | Serving Size *, thou (mL) | Other Ingredients |
|---|---|---|---|---|---|
| Soy Sentials® | 31.one ± one.iv c | half dozen.8 ± 0.3 ab | 10 | 22 (240) | 20 mg isoflavones, 2.5 grand protective blend, LunaRich soy pulverization, cherry-red clover, wild Mexican yam, green tea extract (marketed as a protective supplement for women) |
| Slimplicity® | 26.3 ± 1.8 cd | vii.1 ± 0.5 ab | 10 | 27 (240) | Conjugated linoleic acid, LunaRich soy powder, Advantra Z, L-carnitine, inulin, ChromeMate, CitriMax, Optizinc (marketed as meal replacement shake) |
| Now® | 23.half dozen ± 0.six d | four.4 ± 0.1 c | 7 | eighteen.76 (240) | 197 mg proprietary blend, LunaRich soy pulverisation, vitamins and minerals |
| Provantage® | 23.3 ± 1.i d | 6.1 ± 0.three b | fourteen | 26 (240) | 694 mg amino acid blend, 1220 mg performance blend, LunaRich soy powder, Tonalin, medium-chain triglycerides, creatine, CoQ10 (marketed equally a dietary supplement for sports diet) |
| LunaRich X™ | 41.0 ± two.ane b | 0.2 ± 0.0 d | --- | 0.4 (capsule) | 125 mg soy bioactive lunasin peptide (concentrated class of lunasin) |
| GlucAffect® | 56.4 ± 0.vi a | half dozen.8 ± 0.i ab | 5 | 12 (240) | fifteen mg pycnogenol, 2221 mg proprietary blend, LunaRich soy pulverisation, omega-3-fish oils, Salacia extract, Glucohelp (marketed equally a dietary supplement for maintaining healthy blood sugar) |
| Now® for Kids (Vanilla) | 26.ii ± 1.1 d | 7.6 ± 0.3 a | v | 29 (240) | 408 mg proprietary alloy, LunaRich soy pulverisation, omega-3 fatty acid, phosphatidylserine, phosphatidylcholine, grape seed excerpt (marketed to aid kids heave free energy and mental performance) |
| Now® for Kids (Chocolate) | 25.i ± three.four d | 7.3 ± i.0 a | 5 | 29 (240) | 408 mg proprietary alloy, LunaRich soy powder, omega-3 fatty acid, phosphatidylserine, phosphatidylcholine, grape seed excerpt (marketed to help kids heave free energy and mental performance) |
Table 2. Protein kinase B (AKT) pathway poly peptide expression of treated melanoma cells with ICl compared to untreated control (100% expression).
Table 2. Protein kinase B (AKT) pathway protein expression of treated melanoma cells with IC50 compared to untreated control (100% expression).
| Abbreviation | Full Name | Expression (%) | Epitope |
|---|---|---|---|
| B16-F10 | |||
| AKT | Protein B kinase | 57 | Ser473 |
| AMPKa | AMP-activated protein kinase | 59 | Thr172 |
| BAD | BCL2-associated agonist of cell decease | 51 | Ser112 |
| 4E-BP1 | 4E-binding poly peptide ane | 68 | Thr36 |
| ERK 1/ii | Extracellular point-regulated kinase | 72 | ERK1 Thr202/Tyr204 ERK2 Tyr185/Tyr187 |
| GSK3b | Glycogen synthase kinase 3b | 119 | Ser9 |
| mTOR | Mammalian target of rapamycin | 54 | Ser2448 |
| PTEN | Phosphatase and tensin homolog | 56 | Ser380 |
| Raf-i | Rapidly accelerated fibrosarcoma-1 | 59 | Ser301 |
| RPS6 | Ribosomal protein S6 | 52 | Ser235/Ser236 |
| RSK1 | Ribosomal S6 kinase 1 | 50 | Ser380 |
| A-375 | |||
| Akt | Protein B kinase | 137 | Ser 473 |
| GSK3a | Glycogen synthase kinase 3a | 155 | Ser21 |
| RPS6 | Ribosomal protein S6 | 124 | Ser380 |
| Publisher'south Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and weather of the Artistic Eatables Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
thieletoeopla1999.blogspot.com
Source: https://www.mdpi.com/2072-6643/13/5/1618/htm
0 Response to "Current State of Art After Twenty Years of the Discovery of Bioactive Peptide Lunasin"
Enregistrer un commentaire